A chemistry lesson
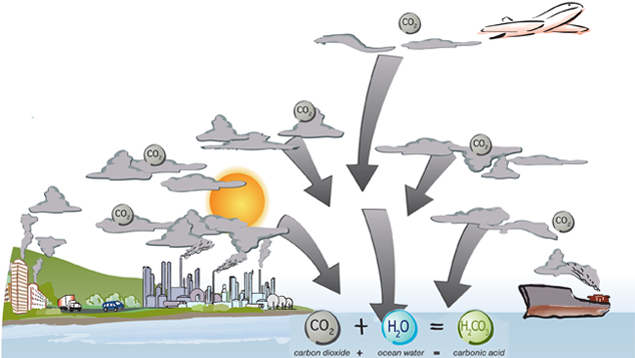
The ocean is one of the largest carbon dioxide (CO2) sinks on the planet. It naturally absorbs CO2 from the atmosphere and can store it for thousands of years. However, since the Industrial Revolution the ocean been trying to keep up with the extra emissions from humans. Here’s the chemistry lesson:
When CO2 is absorbed by the ocean it reacts with water (H2O) to form carbonic acid (H2CO3). CO2 + H2O = H2CO3
The carbonic acid then breaks apart into hydrogen ions (H+) and bicarbonate ions (HCO3-). This extra hydrogen decreases the pH (i.e. increases the acidity) of the surrounding seawater. H2CO3 ↔ HCO3- + H+
The hydrogen ions bind with naturally occurring carbonate in the water to form more bicarbonate. This carbonate would otherwise be available for marine organisms to make their shells and skeletons. H+ + CO3- ↔ HCO3-
While it sounds complicated, the result is a matter of simple chemistry. It is observable and predictable. The ocean’s acidity has increased 30 percent since pre-industrial times. Scientists predict that it will increase 150-185 percent by the end of the century if we don’t reduce our CO2 emissions.






